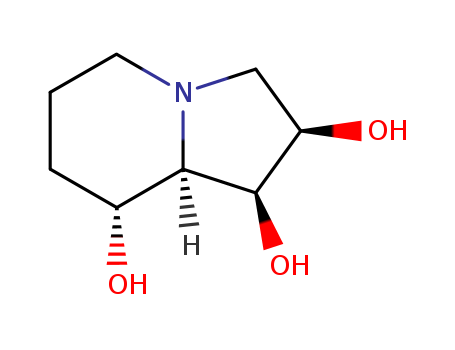
- Chemical Name:Swainsonine
- CAS No.:72741-87-8
- Molecular Formula:C8H15NO3
- Molecular Weight:173.212
- Hs Code.:29339900
- European Community (EC) Number:615-797-6
- NSC Number:614553
- UNII:RSY4RK37KQ
- DSSTox Substance ID:DTXSID5046356
- Nikkaji Number:J12.698E
- Wikipedia:Swainsonine
- Wikidata:Q415324
- NCI Thesaurus Code:C152742
- Pharos Ligand ID:8KU45WX6YPXL
- Metabolomics Workbench ID:53458
- ChEMBL ID:CHEMBL371197
- Mol file:72741-87-8.mol
Synonyms:Swainsonine;Swainsonine, (1R-(2 beta,8a alpha))-Isomer;Swainsonine, (2 beta,8a alpha)-Isomer;Swainsonine, (8 alpha)-Isomer;Swainsonine, (8 alpha,8a alpha)-Isomer;Swainsonine, (8a alpha)-Isomer


 Xn
Xn

