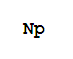Chemical Property of Neptunium
Edit
Chemical Property:
- Melting Point:637°
- Boiling Point:bp 4174°
- PSA:0.00000
- Density:20.45; d 19.36
- LogP:0.00000
- Storage Temp.:-20°C
- Solubility.:Soluble in DMSO (>25 mg/ml)
- Water Solubility.:soluble HCl [HAW93]
- Hydrogen Bond Donor Count:0
- Hydrogen Bond Acceptor Count:0
- Rotatable Bond Count:0
- Exact Mass:237.04817
- Heavy Atom Count:1
- Complexity:0
- Purity/Quality:
-
99%, *data from raw suppliers
NEPTUNIUM 95.00% *data from reagent suppliers
Safty Information:
- Pictogram(s):
- Hazard Codes:
- MSDS Files:
-
SDS file from LookChem
Useful:
- Chemical Classes:Physical/Radiation -> Radionuclides
- Canonical SMILES:[Np]
-
Description
ML300 is a selective non-covalent inhibitor of SARS-CoV 3CL protease (IC50 = 4.11 μM).1,2
-
Physical properties
The chemistry of neptunium (93Np) is somewhat similar to that of uranium (92U) and plutonium(94Pu), which immediately precede and follow it in the actinide series on the periodictable. The discovery of neptunium provided a solution to a puzzle as to the missing decayproducts of the thorium decay series, in which all the elements have mass numbers evenlydivisible by four; the elements in the uranium series have mass numbers divisible by fourwith a remainder of two. The actinium series elements have mass numbers divisible by fourwith a remainder of three. It was not until the neptunium series was discovered that a decayseries with a mass number divisible by four and a remainder of one was found. The neptuniumdecay series proceeds as follows, starting with the isotope plutonium-241: Pu-241→Am-241→Np-237→Pa-233→U-233→Th-229→Ra-225→Ac-225→Fr-221→At-217→Bi-213→Ti-209→Pb-209→Bi-209.Neptunium is a silvery-white radioactive, heavy metal. Its melting point is 644°C, its boilingpoint is 3,902°C, and its density is 20.25g/cm3.
-
Uses
Source material for production of 238U (power source). The most important radioactive isotope of neptunium is Neptunium-237, with a half-lifeof 2.144×10+6years, or about 2.1 million years, and decays into protactinium-233 throughalpha decay. Neptunium’s most important use is in nuclear research and for instrumentsdesigned to detect neutrons.