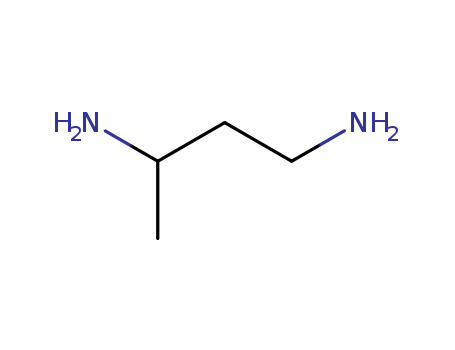
- Chemical Name:1,3-Butanediamine
- CAS No.:590-88-5
- Molecular Formula:C4H12 N2
- Molecular Weight:88.1527
- Hs Code.:
- European Community (EC) Number:209-692-0
- ICSC Number:1078
- NSC Number:13184
- UN Number:2733
- UNII:Z1263602CV
- DSSTox Substance ID:DTXSID20870641
- Nikkaji Number:J35.125C
- Wikidata:Q1932398
- Mol file:590-88-5.mol
Synonyms:1,3-Butanediamine;1,3-Diaminobutane;butane-1,3-diamine;590-88-5;1-Methyltrimethylenediamine;CCRIS 6677;EINECS 209-692-0;NSC 13184;BRN 0605281;AI3-52308;NSC-13184;Z1263602CV;NSC13184;1-methyl-1,3-propanediamine;WLN: ZY1&2Z;UNII-Z1263602CV;DTXSID20870641;AKOS005359739;(+/-)-BUTANE-1,3-DIAMINE;J35.125C;LS-45639;EN300-146371;Q1932398