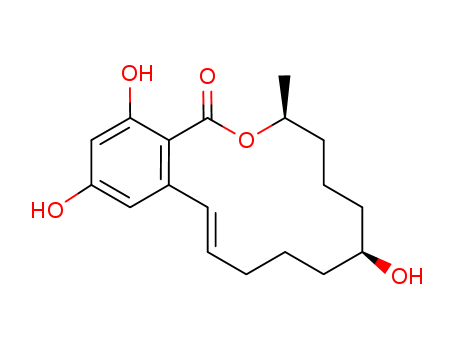
Technology Process of (4S,8S)-8,16,18-trihydroxy-4-methyl-3-oxabicyclo[12.4.0]octadeca-1(14),12,15,17-tetraen-2-one
There total 29 articles about (4S,8S)-8,16,18-trihydroxy-4-methyl-3-oxabicyclo[12.4.0]octadeca-1(14),12,15,17-tetraen-2-one which
guide to synthetic route it.
The literature collected by LookChem mainly comes from the sharing of users and the free literature resources found by Internet computing technology. We keep the original model of the professional version of literature to make it easier and faster for users to retrieve and use. At the same time, we analyze and calculate the most feasible synthesis route with the highest yield for your reference as below:
synthetic route:
- Guidance literature:
-
With
hydrogenchloride;
In
tetrahydrofuran;
at 40 ℃;
DOI:10.1039/b005942k
-
![(3S,7S,E)-14,16-dimethoxy-7-(4-methoxybenzyloxy)-3-methyl-3,4,5,6,7,8,9,10-octahydro-1H-benzo[c][1]oxacyclotetradecin-1-one](/Databaselist/images/loading.webp)
-
(3S,7S,E)-14,16-dimethoxy-7-(4-methoxybenzyloxy)-3-methyl-3,4,5,6,7,8,9,10-octahydro-1H-benzo[c][1]oxacyclotetradecin-1-one
- Guidance literature:
-
With
iodine; tetra-(n-butyl)ammonium iodide; aluminium;
In
benzene;
at 20 ℃;
Inert atmosphere;
DOI:10.1080/00397911.2017.1383433
- Guidance literature:
-
With
dipotassium hydrogenphosphate; sodium chloride;
In
N,N-dimethyl-formamide;
glucose, yeast extract, neopeptone, Mucor bainieri NRRL 2988;
DOI:10.1021/jo00238a008


 Xn
Xn

