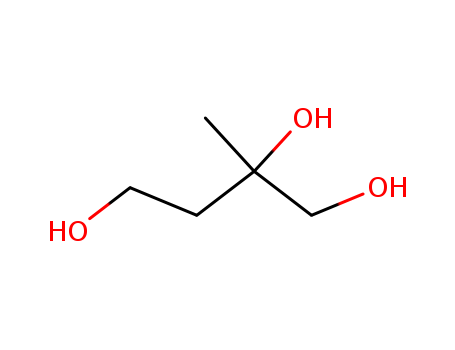
- Chemical Name:2-Methylbutane-1,2,4-triol
- CAS No.:62875-07-4
- Molecular Formula:C5H12O3
- Molecular Weight:120.148
- Hs Code.:
- European Community (EC) Number:263-746-8
- DSSTox Substance ID:DTXSID80978612
- Nikkaji Number:J307.141C
- Mol file:62875-07-4.mol
Synonyms:2-Methylbutane-1,2,4-triol;62875-07-4;1,2,4-Butanetriol, 2-methyl-;EINECS 263-746-8;2-methyl-1,2,4-butanetriol;1,2,4-Butanetriol,2-methyl-;SCHEMBL28594;DTXSID80978612;MFCD18976145;AKOS024257954;SB84538;AS-58672;EN300-1706111;Z1238572721