10.1016/0008-6215(83)88099-9
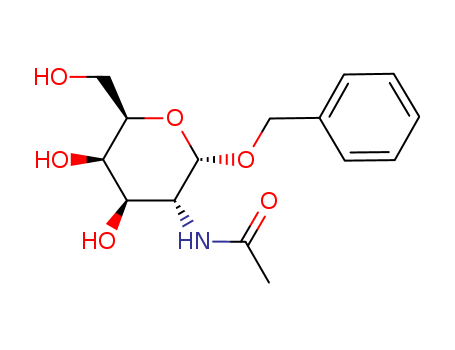
The research aimed to synthesize benzyl 2,3,4-tri-acetamido-2,3,4,6-tetradeoxy-α-D-galactopyranoside, a compound with D-galacto configuration, which was required for other work in the laboratory. The synthesis process involved several steps, starting with the treatment of benzyl 2-acetamido-2-deoxy-α-D-allopyranoside with methanesulfonyl chloride in pyridine to produce a tri-O-mesyl derivative. This was then reduced with sodium borohydride to form benzyl 2-acetamido-2,6-dideoxy-3,4-di-O-methylsulfonyl-α-D-allopyranoside. Further transformations through catalytic reduction and N-acetylation led to the target compound. The process also included the removal of the aglycon from the synthesized compound to obtain the alditol, which was then reduced and acetylated. The conclusions were supported by the 'H-n.m.r. and 13C-n.m.r. spectra, which confirmed the structure of the synthesized compounds, and the mass spectrum of the final product, which showed a single peak, indicating the successful synthesis of the desired compound.


 Xi
Xi


