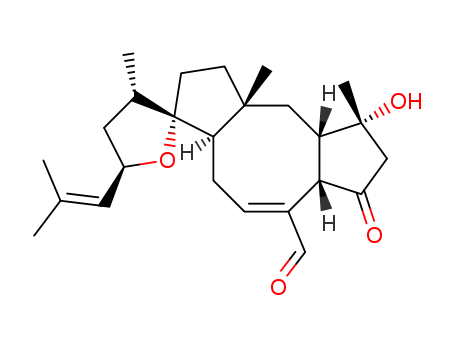
Multi-step reaction with 23 steps
1.1: diethyl ether / 2.5 h / -78 - 0 °C / Inert atmosphere
2.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C / Inert atmosphere
2.2: -78 - 20 °C / Inert atmosphere
3.1: potassium tert-butylate / tetrahydrofuran / 1 h / 20 °C / Inert atmosphere
3.2: 0 °C / Inert atmosphere
4.1: pyridinium p-toluenesulfonate / ethanol / 20 °C / Inert atmosphere
5.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 20 °C / Inert atmosphere
6.1: diethyl ether / 0 °C / Inert atmosphere
7.1: Dess-Martin periodane / dichloromethane / 20 °C / Inert atmosphere
8.1: 1H-imidazole / N,N-dimethyl-formamide / 20 °C / Inert atmosphere
9.1: potassium hexamethylsilazane / tetrahydrofuran; toluene / 0.5 h / -78 °C / Inert atmosphere
9.2: -78 °C / Inert atmosphere
10.1: tetrakis(triphenylphosphine) palladium(0); triethylamine / methanol; toluene / 50 °C / Inert atmosphere
11.1: diisobutylaluminium hydride / hexane / -78 °C / Inert atmosphere
12.1: pyridine; dmap / dichloromethane / 20 °C / Inert atmosphere
13.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 20 °C / Inert atmosphere
14.1: dmap; N-ethyl-N,N-diisopropylamine / dichloromethane / 0 °C / Inert atmosphere
15.1: sodium hydride / N,N-dimethyl-formamide / 0 °C / Inert atmosphere
16.1: diisobutylaluminium hydride / hexane / -78 °C / Inert atmosphere
17.1: Hoveyda-Grubbs catalyst second generation; p-benzoquinone / toluene / 110 °C / Inert atmosphere
18.1: sodium hydride / N,N-dimethyl-formamide / 0 °C / Inert atmosphere
19.1: pyridinium p-toluenesulfonate / ethanol / 20 °C / Inert atmosphere
20.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C / Inert atmosphere
20.2: -78 - 20 °C / Inert atmosphere
21.1: n-butyllithium / tetrahydrofuran; hexane / 1 h / -78 °C / Inert atmosphere
21.2: 20 °C / Inert atmosphere
22.1: naphthalene; lithium / tetrahydrofuran / -30 °C / Inert atmosphere
23.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C / Inert atmosphere
23.2: -78 - 20 °C / Inert atmosphere
With
pyridine; 1H-imidazole; dmap; tetrakis(triphenylphosphine) palladium(0); n-butyllithium; Hoveyda-Grubbs catalyst second generation; oxalyl dichloride; naphthalene; potassium tert-butylate; tetrabutyl ammonium fluoride; pyridinium p-toluenesulfonate; lithium; potassium hexamethylsilazane; sodium hydride; diisobutylaluminium hydride; Dess-Martin periodane; dimethyl sulfoxide; triethylamine; N-ethyl-N,N-diisopropylamine; p-benzoquinone; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione;
In
tetrahydrofuran; methanol; diethyl ether; ethanol; hexane; dichloromethane; dimethyl sulfoxide; N,N-dimethyl-formamide; toluene;
2.1: Swern oxidation / 2.2: Swern oxidation / 3.1: Wittig reaction / 3.2: Wittig reaction / 20.1: Swern oxidation / 20.2: Swern oxidation / 21.1: Wittig reaction / 21.2: Wittig reaction / 23.1: Swern oxidation / 23.2: Swern oxidation;
DOI:10.1002/anie.201104447



 Xn
Xn

