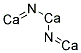Base Information
Edit
- Chemical Name:Calcium nitride (Ca3N2)
- CAS No.:12013-82-0
- Molecular Formula:Ca3N2
- Molecular Weight:148.25
- Hs Code.:
- Mol file:12013-82-0.mol

Synonyms:12013-82-0;Calcium nitride (Ca3N2);calcium;azanidylidenecalcium;Calciumnitrid;Calcium nitride, -200 mesh, 95%;Calcium nitride, powder, -200 mesh, 99% trace metals basis (contains <0.5% Mg)