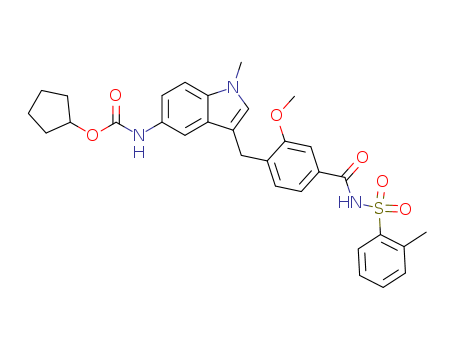
- Chemical Name:Zafirlukast
- CAS No.:107753-78-6
- Molecular Formula:C31H33N3O6S
- Molecular Weight:575.686
- Hs Code.:
- European Community (EC) Number:663-705-8
- UNII:XZ629S5L50
- DSSTox Substance ID:DTXSID5023746
- Nikkaji Number:J322.324H
- Wikipedia:Zafirlukast
- Wikidata:Q928378
- NCI Thesaurus Code:C47785
- RXCUI:114970
- Pharos Ligand ID:GJ5ZBJBGBWFX
- Metabolomics Workbench ID:42885
- ChEMBL ID:CHEMBL603
- Mol file:107753-78-6.mol
Synonyms:4-(5-cyclopentyloxycarbonylamino-2-methylindol-3-yl-methyl)-3-methoxy-N-O-tolylsulfonylbenzamide;Accolate;Aeronix;ICI 204,219;ICI 204219;ICI-204219;Olmoran;zafirlukast