Base Information
Edit
- Chemical Name:vanadium (V) oxide
- CAS No.:1314-62-1
- Molecular Formula:V2O5
- Molecular Weight:181.88
- Hs Code.:2825.30 Oral rat LD50: 10 mg/kg
- UNII:BVG363OH7A
- Mol file:1314-62-1.mol
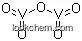
Synonyms:vanadium (V) oxide;VANADIUM PENTOXIDE [MI];VANADIUM PENTOXIDE [HSDB];VANADIUM PENTOXIDE [IARC];XHCLAFWTIXFWPH-UHFFFAOYSA-N;V0169


 Xi,
Xi, N,
N, T
T
