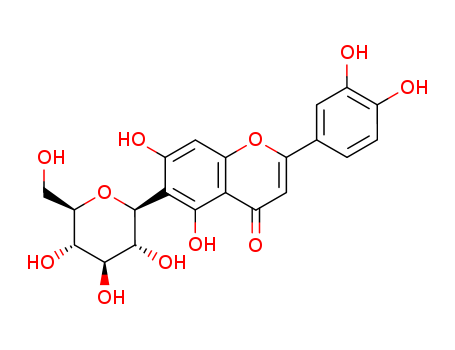
- Chemical Name:HOMOORIENTIN
- CAS No.:4261-42-1
- Molecular Formula:C21H20O11
- Molecular Weight:448.383
- Hs Code.:29389090
- European Community (EC) Number:610-045-3
- UNII:A37342TIX1
- DSSTox Substance ID:DTXSID50962609
- Nikkaji Number:J14.555F
- Wikipedia:Isoorientin
- Wikidata:Q3155592
- Metabolomics Workbench ID:51105
- ChEMBL ID:CHEMBL239559
- Mol file:4261-42-1.mol
Synonyms:Isoorientin(7CI,8CI);3',4',5,7-Tetrahydroxy-6-C-glucopyranosylflavone;Homoorientin;Lespecapitioside;Lespecapitoside;Luteolin 6-C-glucoside;Luteolin 6-C-b-D-glucopyranoside;Luteolin 6-C-b-D-glucoside;Luteolin 6-C-b-glucopyranoside;Lutonaretin;(1S)-1,5-anhydro-1-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-6-yl]-D-glucitol;