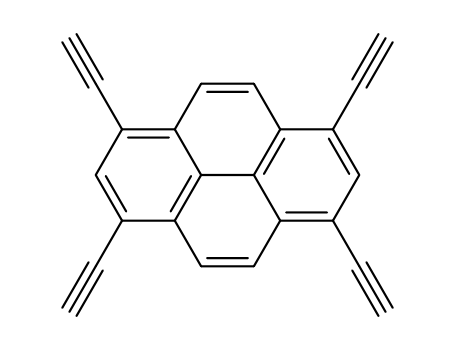
- Chemical Name:1,3,6,8-Tetraethynylpyrene
- CAS No.:870259-02-2
- Molecular Formula:C24H10
- Molecular Weight:298.343
- Hs Code.:
- DSSTox Substance ID:DTXSID30469747
- Nikkaji Number:J2.241.292B
- Wikidata:Q82297599
- Mol file:870259-02-2.mol
Synonyms:1,3,6,8-tetraethynylpyrene;870259-02-2;Pyrene, 1,3,6,8-tetraethynyl-;YSWG351;Pyrene,1,3,6,8-tetraethynyl;DTXSID30469747;MFCD21609448;AKOS040768755;BS-46194;CS-0110386;E74965