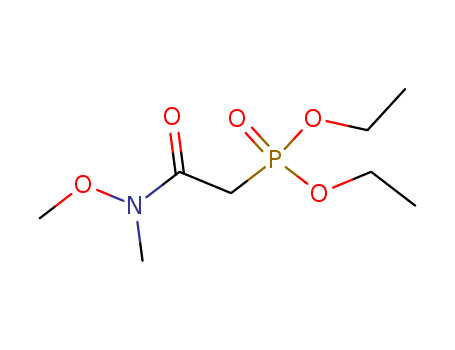
- Chemical Name:2-diethoxyphosphoryl-N-methoxy-N-methylacetamide
- CAS No.:124931-12-0
- Molecular Formula:C8H18 N O5 P
- Molecular Weight:239.208
- Hs Code.:29319000
- European Community (EC) Number:626-937-0
- DSSTox Substance ID:DTXSID70378667
- Nikkaji Number:J860.320K
- Wikidata:Q82168183
- Mol file:124931-12-0.mol
Synonyms:124931-12-0;Diethyl (N-methoxy-N-methylcarbamoylmethyl)phosphonate;2-diethoxyphosphoryl-N-methoxy-N-methylacetamide;Diethyl (2-(methoxy(methyl)amino)-2-oxoethyl)phosphonate;Diethyl(N-methoxy-N-methylcarbamoylmethyl)phosphonate;Diethyl N-methoxy-N-methylphosphonoacetamide;2-diethoxyphosphoryl-N-methoxy-N-methyl-acetamide;diethyl {[methoxy(methyl)carbamoyl]methyl}phosphonate;diethyl {2-[methoxy(methyl)amino]-2-oxoethyl}phosphonate;SBB057815;diethyl (n-methoxy-n-methylcarbamoyl-methyl)phosphonate;SCHEMBL224985;DTXSID70378667;WYLRYBDGIILFIR-UHFFFAOYSA-N;(N-Methoxy-N-methylcarbamoylmethyl)phosphonic Acid Diethyl Ester;ZEA93112;BBL102956;MFCD00134233;STL556765;AKOS015916081;AM803081;N-methoxy-N-methyl diethylphosphonoacetamide;CS-0037993;D3708;N-methoxy-N-methyl diethylphosphonoacetarnide;N-Methoxy-N-methyldiethoxyphosphinylacetamide;EN300-7415618;A890344;N-Methoxy-N-methyl-2-(diethyl phosphono)acetamide;J-005180;N-Methoxy-N-methyl-phosphonoacetamide diethyl ester;diethyl 2-(methoxy(methyl)amino)-2-oxoethylphosphonate;diethyl(N-methoxy-N-methyl-carbamoylmethyl)phosphonate;diethyl-(N-methoxy-N-methyl-carbamoylmethyl)-phosphonate;Diethyl (N-methoxy-N-methylcarbamoylmethyl)phosphonate, 96%



 Xi
Xi
