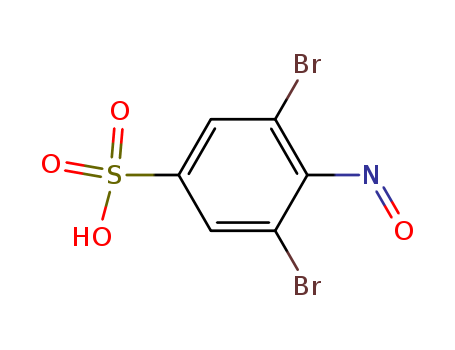
10.1021/j100350a029
The study investigates the sonochemistry of argon-saturated water-alcohol mixtures using ESR and spin trapping with 3,5-dibromo-4-nitrosobenzenesulfonate (DBNBS). It examines free-radical intermediates induced by 50-kHz ultrasound in aqueous solutions of ethanol, 1-propanol, 2-propanol, and 2-methyl-2-propanol. The chemicals involved include various alcohols (ethanol, 1-propanol, 2-propanol, 2-methyl-2-propanol, methanol), water (H2O), deuterated water (D2O), and DBNBS as the spin trap. The alcohols serve as the primary solutes under investigation, while water and deuterated water act as solvents and provide isotopic labeling for tracking reactions. DBNBS is crucial for capturing and identifying radicals formed during sonolysis through its spin adducts. The study identifies spin adducts typical of thermal decomposition of the alcohols and of H- and OH-induced abstraction reactions, observes isotopically mixed radicals in mixed-isotope systems, and examines the effects of solvent composition and temperature on the sonochemical yields of radicals.