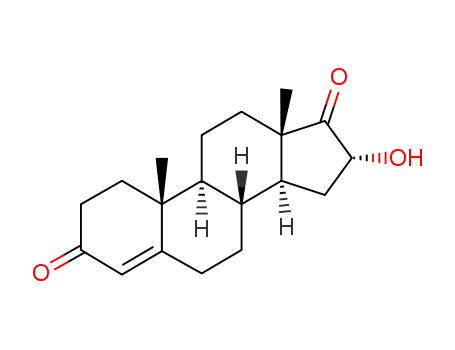
- Chemical Name:16alpha-Hydroxyandrostenedione
- CAS No.:63-02-5
- Molecular Formula:C19H26 O3
- Molecular Weight:302.40794
- Hs Code.:
- UNII:JN8H7214IR
- DSSTox Substance ID:DTXSID20331500
- Nikkaji Number:J337.168I
- Wikipedia:16%CE%B1-Hydroxyandrostenedione
- Wikidata:Q27103209
- Metabolomics Workbench ID:35346
- ChEMBL ID:CHEMBL1908014
- Mol file:63-02-5.mol
Synonyms:16 alpha-hydroxyandrost-4-en-3,17-dione;16-hydroxyandrost-4-en-3,17-dione;16-hydroxyandrost-4-en-3,17-dione, (16alpha)-isomer;16-hydroxyandrost-4-en-3,17-dione, (16beta)-isomer;16alpha-hydroxyandrostenedione;16alpha-OHAD