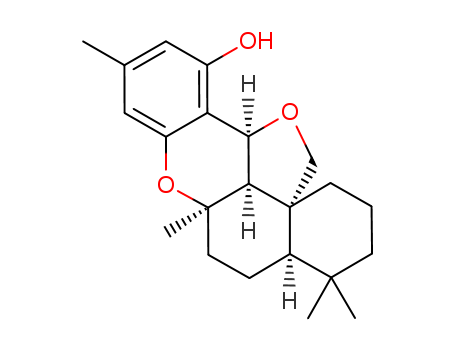
- Chemical Name:Sicanina
- CAS No.:22733-60-4
- Molecular Formula:C22H30O3
- Molecular Weight:342.478
- Hs Code.:
- NSC Number:135048
- DSSTox Substance ID:DTXSID20860284
- ChEMBL ID:CHEMBL1416486
- Mol file:22733-60-4.mol
Synonyms:22733-60-4;Siccanin (TN);NSC135048;NCGC00181099-01;4,4,6a,9-Tetramethyl-1,2,3,4,4a,5,6,6a,11b,13b-decahydrobenzo[a]furo[2,3,4-mn]xanthen-11-ol;SCHEMBL3064856;CHEMBL1416486;DTXSID20860284;NCI60_000788;(13aS)-1,3,4,4a.beta.,5,6,6a,11b.beta.,13b.beta.-Decahydro-4,4,6a.beta.,9-tetramethyl-13H-benzo[a]furo[2,3,4-mn]xanthen-11-ol;(4R)-8,12,16,16-Tetramethyl-3,11-dioxapentacyclo[10.7.1.01,15.04,20.05,10]icosa-5,7,9-trien-6-ol;13H-Benzo[a]furo[2,4-mn]xanthen-11-ol, 1,2,3,4,4a,5,6,6a,11b,13b-decahydro-4,4,6a,9-tetramethyl-;13H-Benzo[a]furo[2,4-mn]xanthen-11-ol, 1,2,3,4,4a,5,6,6a,11b,13b-decahydro-4,4,6a,9-tetramethyl-, [4aS-(4a.alpha.,6a.alpha.,11b.alpha.,13aR*,13b.alpha.)]-;4,4,6a,9-Tetramethyl-1,2,3,4,4a,5,6,6a,11b,13b-decahydro-13H-benzo[a]furo[2,3,4-mn]xanthen-11-ol