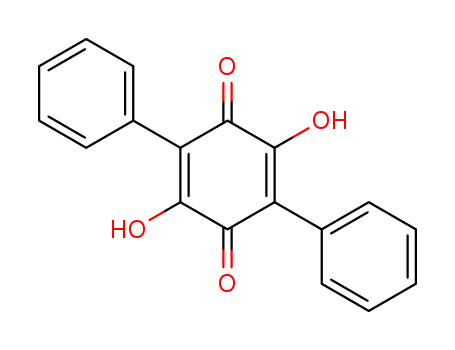
- Chemical Name:2,5-Dihydroxy-3,6-diphenyl-p-benzoquinone
- CAS No.:548-59-4
- Molecular Formula:C18H12O4
- Molecular Weight:292.291
- Hs Code.:2914400090
- NSC Number:44175
- UNII:VM7U3VEH5G
- DSSTox Substance ID:DTXSID50203281
- Nikkaji Number:J11.565G
- Wikipedia:Polyporic_acid
- Wikidata:Q21402073
- Metabolomics Workbench ID:99220
- ChEMBL ID:CHEMBL480678
- Mol file:548-59-4.mol
Synonyms:2,5-dihydroxy-3,6-diphenyl-1,4-benzoquinone;polyporic acid