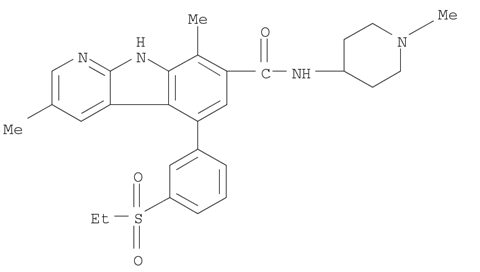
- Chemical Name:5-(3-(ethylsulfonyl)phenyl)-3,8-dimethyl-N-(1-methylpiperidin-4-yl)-9H-pyrido[2,3-b]indole-7-carboxamide
- CAS No.:934541-31-8
- Molecular Formula:C28H32N4O3S
- Molecular Weight:504.653
- Hs Code.:
- UNII:DM9UIR23R7
- ChEMBL ID:CHEMBL3544932
- DSSTox Substance ID:DTXSID70583097
- Metabolomics Workbench ID:153685
- NCI Thesaurus Code:C82674
- Nikkaji Number:J3.408.634F
- Pharos Ligand ID:41TSVPX15U1P
- Wikidata:Q27276478
- Mol file:934541-31-8.mol
Synonyms:TAK-901