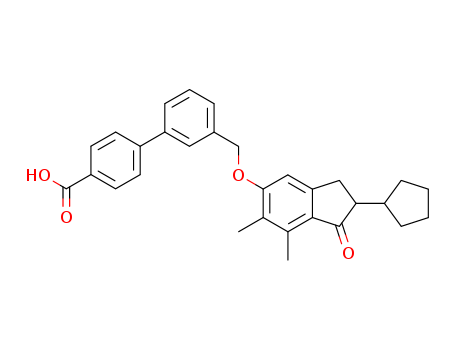
- Chemical Name:Biphenylindanone A
- CAS No.:866823-73-6
- Molecular Formula:C30H30O4
- Molecular Weight:454.566
- Hs Code.:
- DSSTox Substance ID:DTXSID90432098
- Wikipedia:Biphenylindanone_A
- Wikidata:Q4915436
- Pharos Ligand ID:9PVMFDV7CRQS
- Metabolomics Workbench ID:143804
- ChEMBL ID:CHEMBL593013
- Mol file:866823-73-6.mol
Synonyms:3'-(((2-cyclopentyl-6,7-dimethyl-1-oxo-2,3-dihydro-1H-inden-5-yl)oxy)methyl)biphenyl-4-carboxylic acid;BINA cpd;biphenyl-indanone A