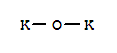Chemical Property of Potassium oxide
Edit
Chemical Property:
- Appearance/Colour:White Microgranular
- Vapor Pressure:24.5mmHg at 25°C
- Melting Point:decomposes at 350℃ [HAW93]
- Boiling Point:100 ºCat 760 mmHg
- Flash Point:°C
- PSA:0.00000
- Density:g/cm3
- LogP:-0.11880
- Water Solubility.:soluble H2O, forming KOH; soluble alcohol and ether [HAW93]
- Hydrogen Bond Donor Count:0
- Hydrogen Bond Acceptor Count:1
- Rotatable Bond Count:0
- Exact Mass:93.92232759
- Heavy Atom Count:3
- Complexity:0
- Purity/Quality:
-
99.9% *data from raw suppliers
Safty Information:
- Pictogram(s):
- Hazard Codes:
- MSDS Files:
-
SDS file from LookChem
Total 1 MSDS from other Authors
Useful:
- Canonical SMILES:[O-2].[K+].[K+]
-
Uses
Potassium oxide is a strong alkaline flux which is similar to sodium oxide but is slightly less strong and it begins its fluxing action earlier than does sodium oxide, at approximately 1382°F (750°C). It's a predictable, stable flux that produces bright glossy glazes, but it can't be used alone as a flux. Potassium produces slightly stronger glaze surfaces than does sodium oxide. Its low viscosity and surface tension create fluid glaze melts, but its high coefficient of expansion and contraction may cause crazing.
As mentioned above, potassium oxide is often found combined with sodium oxide, so it's often written as KNaO. lt's only slightly volatile at ceramic temperatures and is just slightly soluble.Usually used in its insoluble forms as feldspars or slightly soluble frits, potassium oxide can also be introduced to the glaze recipe as soluble pearl ash (potassium carbonate), which can cause some flashing like sodium carbonate.
Insoluble sources of potassium oxide are potash feldspars such as Custer, G-200, K-200, A-3, Kona F-4(Del Monte), Cornwall stone, Plastic Vitrox, volcanic ash, Kona A-1, Bell, Eureka, A-300, and mica. All soda feldspars have some potassium oxide; frits P-25, 3110, and 3124 contain minor amounts. Soluble forms of potassium oxide include pearl ash(K2CO3), potassium nitrate (saltpeter), and unwashed wood ash.