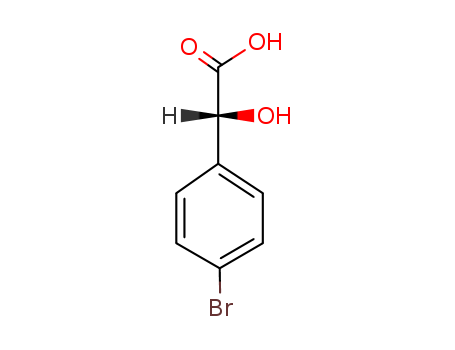
- Chemical Name:4-BROMOMANDELIC ACID
- CAS No.:7021-04-7
- Molecular Formula:C8H7BrO3
- Molecular Weight:231.04300
- Hs Code.:
- Mol file:7021-04-7.mol
Synonyms:(R)-p-bromo-mandelic acid;(-)-4-Bromomandelic acid;p-Bromomandelic acid,(-);Benzeneacetic acid, 4-bromo-α-hydroxy-, (±)-;4-Brom-(R)-(-)-mandelsaeure;(R)-(-)-4-bromomandelic acid;D-4-bromomandelic acid;(R)-4-Brom-mandelsaeure;


 Xi
Xi


