Multi-step reaction with 24 steps
1: 97 percent / LiAlH4 / diethyl ether / 0 °C
2: 70 percent / pyridinium chlorochromate, NaOAc / CH2Cl2 / 2 h
3: 1) lithium diisopropylamide / 1) THF, -78 deg C, 45 min, 2a) THF, -78 deg C, 45 min, 2b) r.t., 5 h
4: 1) MeI, 2) 5 percent aq. NaHCO3 / 1a) MeOH, 4 deg C, overnight, 1b) r.t., 5 h, 2) CH2Cl2, r.t., 16 h
5: 95 percent / CeCl3*6H2O, NaBH4 / methanol / 0 °C / 5 to 10 min
6: 1a) MS 3 Angstroem, diisopropyl (-)-tartrate, 1b) Ti(O-i-Pr)4, 2) cumene hydroperoxide / 1a) CH2Cl2, r.t., 1b) -5 deg C, 20 min, 2a) -5 deg C, 8 h, 2b) 4 deg C, overnight
7: 51 percent / Ti(O-i-Pr)4 / benzene / 0.33 h
8: 98 percent / camphorsulfonic acid / CH2Cl2 / 5 h
9: 90 percent / LiAlH4 / diethyl ether / 1 h / Heating
10: 67 percent / pyridinium dichromate / dimethylformamide / 30 h / Ambient temperature
11: 99 percent / diethyl ether
12: 93 percent / Ti(OEt)4 / 60 h / 100 °C
13: 1) O3, 2) Me2S / 1) CH2Cl2, -78 deg C, 45 min, 2) to r.t., 7 h
14: 67 percent / NaIO4, 2 percent RuCl3*3H2O / acetonitrile; CCl4 / 0.33 h / Ambient temperature; phosphate buffer, pH 7
15: 96 percent / HOAc / H2O / 8 h / 75 °C
16: 74 percent / 2-chloro-1-methylpyridinium iodide, 4-(dimethylamino)pyridine / acetonitrile / a) 0 deg C, 3 h, b) r.t., 10 h
17: 91 percent / (i-Pr)2NEt / tetrahydrofuran / 15 h / 50 °C
18: 1) N-isopropylcyclohexylamine, n-BuLi / 1) THF, hexane, -60 to -50 deg C, 45 min, 2a) -60 deg C, 45 min, 2b) to -20 deg C, 10 min
19: Et3N, 4-(dimethylamino)pyridine / CH2Cl2 / 6 h
20: 1,8-diazabicyclo<5.4.0>undec-7-ene / CH2Cl2 / 15 h / 4 °C
21: 90 percent / 3 M aq. HCl / tetrahydrofuran / a) r.t., 1 h, b) 35 deg C, 10 h
22: 60 percent / RuCl3*3H2O, 30 percent aq. H2O2 / tetrahydrofuran / a) 0 deg C, 0.5 h, b) r.t., 2 h
23: 91 percent / camphorsulfonic acid / CHCl3 / 12 h / Ambient temperature
With
titanium(IV) isopropylate; hydrogenchloride; dmap; ruthenium trichloride; titanium(IV) tetraethanolate; sodium tetrahydroborate; sodium periodate; lithium aluminium tetrahydride; dipyridinium dichromate; n-butyllithium; cerium(III) chloride; (-)-diisopropyl tartrate; dimethylsulfide; Cumene hydroperoxide; MS 3 Angstroem; N-cyclohexylisopropylamine; camphor-10-sulfonic acid; 2-chloro-1-methyl-pyridinium iodide; dihydrogen peroxide; sodium acetate; sodium hydrogencarbonate; ozone; acetic acid; 1,8-diazabicyclo[5.4.0]undec-7-ene; triethylamine; N-ethyl-N,N-diisopropylamine; pyridinium chlorochromate; lithium diisopropyl amide; methyl iodide;
In
tetrahydrofuran; methanol; tetrachloromethane; diethyl ether; dichloromethane; chloroform; water; N,N-dimethyl-formamide; acetonitrile; benzene;
DOI:10.1021/jo00034a017


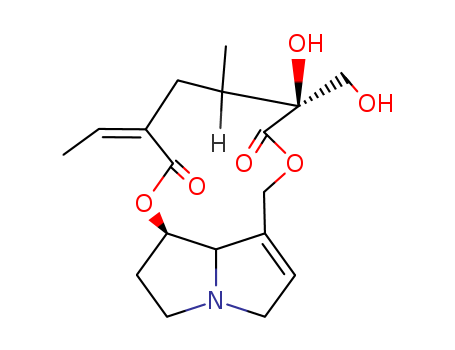
 A poison.:;
A poison.:;
