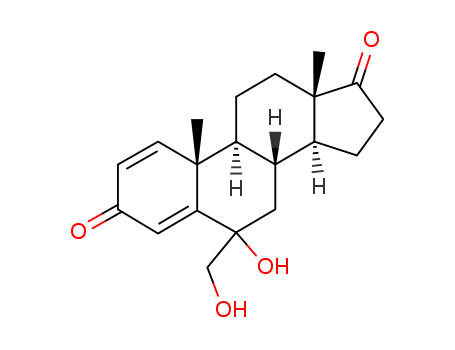
- Chemical Name:FCE 27473
- CAS No.:184972-11-0
- Molecular Formula:C20H26O4
- Molecular Weight:330.424
- Hs Code.:
- Mol file:184972-11-0.mol
Synonyms:6-Hydroxy-6-(hydroxyMethyl)-androsta-1,4-diene-3,17-dione;FCE 27473;6-Hydroxy-6-(hydroxyMethyl)-androsta-1,4-diene-3,17-dione (Mixture of DiastereoMers)