Base Information
Edit
- Chemical Name:Calcium Sulfate Dihydrate
- CAS No.:13397-24-5
- Deprecated CAS:77030-59-2,70513-70-1
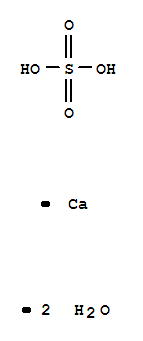
- Molecular Formula:CaSO4.2H2O
- Molecular Weight:154.16
- Hs Code.:
- European Community (EC) Number:600-148-1,927-377-2
- ICSC Number:1734
- UNII:4846Q921YM
- DSSTox Substance ID:DTXSID7047514
- Wikipedia:Alabaster
- Wikidata:Q30135771
- NCI Thesaurus Code:C65281
- RXCUI:1310465
- Mol file:13397-24-5.mol
Synonyms:Alabaster;Anhydrous Sulfate of Lime;Artificial Dental Stone;Calcium Sulfate;Calcium Sulfate (1:1), Dihydrate;Calcium Sulfate (1:1), Hemihydrate;Calcium Sulfate (2:1);Calcium Sulfate Dihydrate;Calcium Sulfate, Anhydrous;Calcium Sulfate, Dihydrate;Calcium Sulfate, Hemihydrate;Calcium Sulphate;Dental Gypsum;Dental Stone, Artificial;Drierite;Gypsite;Gypsum;Gypsum, Dental;Karstenite;Plaster of Paris;Stone, Artificial Dental


 T,
T, N
N
