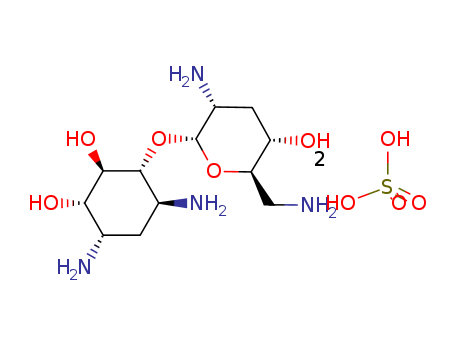
- Chemical Name:NebraMine Disulfate
- CAS No.:71122-29-7
- Molecular Formula:C12H26N4O5*2H2O4S
- Molecular Weight:502.521
- Hs Code.:
- Mol file:71122-29-7.mol
Synonyms:NebraMine Disulfate;2-Deoxy-4-O-(2,6-diaMino-2,3,6-trideoxy-α-D-ribo-hexopyranosyl)-D-streptaMine Disulfate;3'-DeoxyneaMin Disulfate;3'-DeoxyneaMine Disulfate;3'-DeoxyneoMycin A Disulfate;NebraMycin VIII Disulfate;TobraMine Disulfate