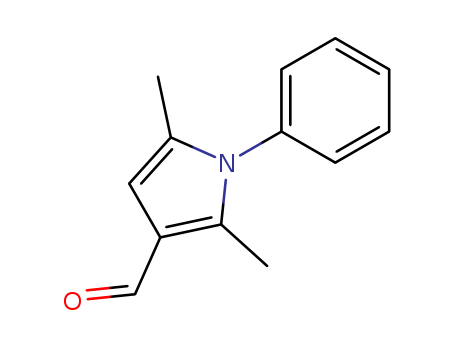
- Chemical Name:2,5-Dimethyl-1-phenyl-1H-pyrrole-3-carbaldehyde
- CAS No.:83-18-1
- Deprecated CAS:1989657-82-0
- Molecular Formula:C13H13 N O
- Molecular Weight:199.252
- Hs Code.:2933990090
- European Community (EC) Number:201-458-6
- NSC Number:68230
- UNII:PW8ELR8SUD
- DSSTox Substance ID:DTXSID2058887
- Nikkaji Number:J192.606C
- Wikidata:Q72471406
- ChEMBL ID:CHEMBL2113933
- Mol file:83-18-1.mol
Synonyms:83-18-1;2,5-Dimethyl-1-phenyl-1H-pyrrole-3-carbaldehyde;2,5-Dimethyl-1-Phenylpyrrole-3-Carboxaldehyde;2,5-dimethyl-1-phenylpyrrole-3-carbaldehyde;1H-Pyrrole-3-carboxaldehyde, 2,5-dimethyl-1-phenyl-;PW8ELR8SUD;EINECS 201-458-6;NSC 68230;NSC-68230;Pyrrole-3-carboxaldehyde, 2,5-dimethyl-1-phenyl-;NSC68230;2,5-dimethyl-1-phenyl-1H-Pyrrole-3-carboxaldehyde;UNII-PW8ELR8SUD;SCHEMBL645648;OSM-S-28;CHEMBL2113933;DTXSID2058887;MFCD00051494;STK297810;AKOS000113216;AM83083;AS-66693;BB 0237624;CS-0186511;FT-0610426;EN300-01173;10.14272/LNROIXNEIZSESG-UHFFFAOYSA-N;2,5-dimethyl-1-phenyl-3-pyrrolecarboxaldehyde;3-FORMYL-2,5-DIMETHYL-1-PHENYLPYRROLE;Pyrrole-3-carboxaldehyde,5-dimethyl-1-phenyl-;W18717;2,5-Dimethyl-1-phenyl pyrrole-3-carboxaldehyde;2,5-dimethyl-1-phenyl-1H-pyrrol-3-carbaldehyd;1-Phenyl-2,5-dimethyl-1H-pyrrole-3-carbaldehyde;10.14272/LNROIXNEIZSESG-UHFFFAOYSA-N.1;1H-Pyrrole-3-carboxaldehyde,5-dimethyl-1-phenyl-;doi:10.14272/LNROIXNEIZSESG-UHFFFAOYSA-N;2,5-Dimethyl-1-phenyl-1H-pyrrole-3-carbalde hyde;doi:10.14272/LNROIXNEIZSESG-UHFFFAOYSA-N.1;W-203889;Z56759012;F1029-0024;2,5-Dimethyl-1-phenyl-1H-pyrrole-3-carbaldehyde, AldrichCPR;4-(5-{[(3-chloro-4-fluorophenyl)sulfonyl]amino}-1-methyl-1H-benzimidazol-2-yl)-N-ethylbenzamide