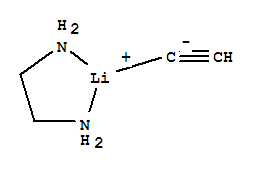
- Chemical Name:Lithium acetylide ethylenediamine complex
- CAS No.:6867-30-7
- Molecular Formula:C4H9 Li N2
- Molecular Weight:92.07
- Hs Code.:38249095
- Mol file:6867-30-7.mol
Synonyms:Ethylenediamine,compd. with lithium acetylide (Li(HC2)) (1:1) (8CI); Lithium,(1,2-ethanediamine-N,N')ethynyl-; Lithium, (1,2-ethanediamine-kN,kN')ethynyl- (9CI); Lithium acetylide (Li(HC2)), compd.with ethylenediamine (1:1) (8CI); (1,2-Diaminoethane)(ethynyl)lithium;(1,2-Ethanediamine-N,N')ethynyllithium; (Acetylido)(ethylenediamine)lithium;Ethylenediamine compd. with Li acetylide; Ethynyl lithium-ethylene diaminecomplex; Ethynyllithium complex with 1,2-ethanediamine (1:1); Lithiumacetylide-ethylenediamine (1:1); Lithium acetylide-ethylenediamine complex