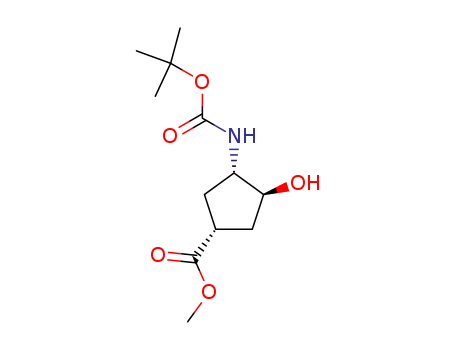
- Chemical Name:(1S,2S,4R)-N-Boc-1-amino-2-hydroxycyclopentane-4-carboxylic acid methyl ester
- CAS No.:262280-14-8
- Molecular Formula:C12H21NO5
- Molecular Weight:259.302
- Hs Code.:29221985
- DSSTox Substance ID:DTXSID20427437
- Nikkaji Number:J1.562.825A
- Wikidata:Q82240095
- Mol file:262280-14-8.mol
Synonyms:262280-14-8;(1S,2S,4R)-N-Boc-1-amino-2-hydroxycyclopentane-4-carboxylic acid methyl ester;(1R,3S,4S)-Methyl 3-((tert-butoxycarbonyl)amino)-4-hydroxycyclopentanecarboxylate;methyl (1R,3S,4S)-3-hydroxy-4-[(2-methylpropan-2-yl)oxycarbonylamino]cyclopentane-1-carboxylate;methyl (1R,3S,4S)-3-{[(tert-butoxy)carbonyl]amino}-4-hydroxycyclopentane-1-carboxylate;Cyclopentanecarboxylic acid, 3-[[(1,1-dimethylethoxy)carbonyl]amino]-4-hydroxy-, methyl ester, (1R,3S,4S)-;SCHEMBL3546943;DTXSID20427437;MFCD02259721;BS-42766;CS-0049306;P17295;Methyl (1R,3S,4S)-3-(Boc-amino)-4-hydroxycyclopentane-1-carboxylate;(1R,3S,4S)-Methyl3-((tert-butoxycarbonyl)amino)-4-hydroxycyclopentanecarboxylate;Methyl (1R,3S,4S)-3-[(t-butoxy)carbonyl]amino-4-hydroxycyclopentane-1-carboxylate;(1R,3S,4S)-3-[[(1,1-Dimethylethoxy)carbonyl]amino]-4-hydroxycyclopentanecarboxylic Acid Methyl Ester;3alpha-[(tert-Butyloxycarbonyl)amino]-4beta-hydroxycyclopentane-1alpha-carboxylic acid methyl ester;METHYL (1R,3S,4S)-3-{[(TERT-BUTOXY)CARBONYL]AMINO-4-HYDROXYCYCLOPENTANE-1-CARBOXYLATE