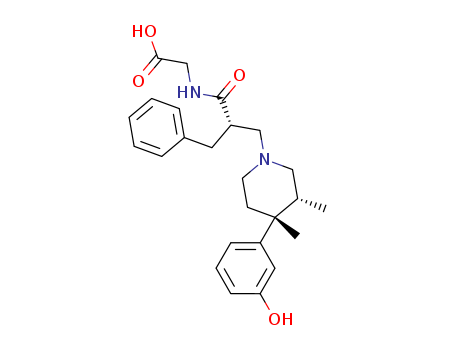
- Chemical Name:Alvimopan
- CAS No.:156053-89-3
- Molecular Formula:C25H32N2O4
- Molecular Weight:424.54
- Hs Code.:2933399090
- European Community (EC) Number:814-137-6
- UNII:Q153V49P3Z
- DSSTox Substance ID:DTXSID60166035
- Nikkaji Number:J605.686E
- Wikipedia:Alvimopan
- Wikidata:Q4738021
- NCI Thesaurus Code:C77377
- RXCUI:1516803
- Pharos Ligand ID:KN3BKG2Q1YWS
- Metabolomics Workbench ID:43667
- ChEMBL ID:CHEMBL270190
- Mol file:156053-89-3.mol
Synonyms:ADL 8-2698;ADL8-2698;alvimopan;alvimopan anhydrous;anhydrous alvimopan;Entereg;LY 246736;LY-246736;LY246736;trans-3,4-dimethyl-4-(3-hydroxyphenyl) piperidine