10.1246/bcsj.52.1459
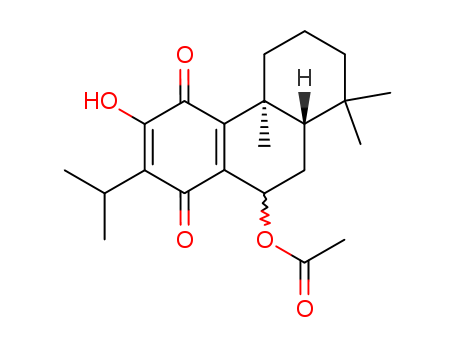
Takashi Matsumoto and Shogo Harada details the total synthesis of several naturally occurring tricyclic diterpenes with an abietane skeleton. The authors describe the synthesis of (+)-taxoquinone, (-)-7a-acetoxyroyleanone, (-)-dehydroroyleanone, (-)-horminone, (-)-7-oxoroyleanone, and (+)-inuroyleanol, all starting from (+)-ferruginol. The synthesis involves various chemical transformations, including methylation, oxidation with chromium trioxide, reduction with sodium borohydride, acetylation, and other reactions. The structures of the synthesized compounds were confirmed through NMR spectroscopy and other analytical methods. The study represents a significant advancement in the field of natural product synthesis, providing detailed procedures and characterizations for these complex diterpenes.