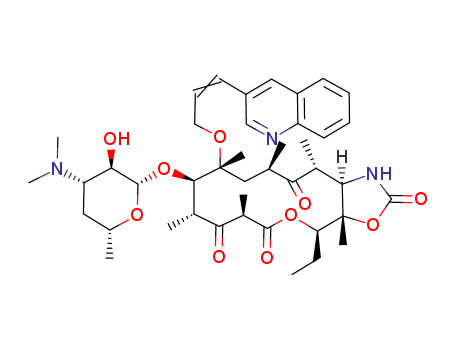
- Chemical Name:Abbott-195773
- CAS No.:205110-48-1
- Molecular Formula:C42H59N3O10
- Molecular Weight:765.945
- Hs Code.:
- DSSTox Substance ID:DTXSID00870225
- Wikipedia:Cethromycin
- NCI Thesaurus Code:C83613
- Metabolomics Workbench ID:149770
- ChEMBL ID:CHEMBL3989904
- Mol file:205110-48-1.mol
Synonyms:A 195,773;A 195773;A-195,773;A-195773;ABT 773;ABT-773;cethromycin