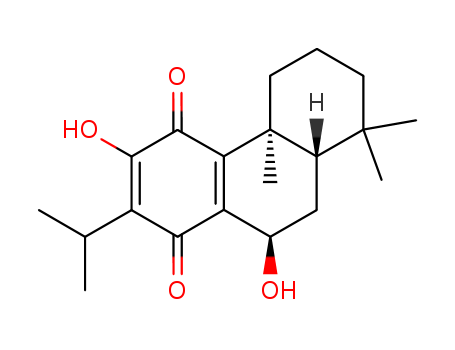
- Chemical Name:Horminone
- CAS No.:21887-01-4
- Molecular Formula:C20H28O4
- Molecular Weight:332.44
- Hs Code.:
- UNII:OR166S9YJA
- DSSTox Substance ID:DTXSID401318668
- Nikkaji Number:J16.504B
- Wikidata:Q27140327,Q104394457
- Metabolomics Workbench ID:66268
- ChEMBL ID:CHEMBL517846
- Mol file:21887-01-4.mol
Synonyms:4b,5,6,7,8,8a,9,10-octahydro-3,10-dihydroxy-4b,8,8-trimethyl-2-(1-methylethyl)-1,4-phenanthrenedione;7alpha-12-hydroxy-8,12-abietadiene-11,14-dione;horminone