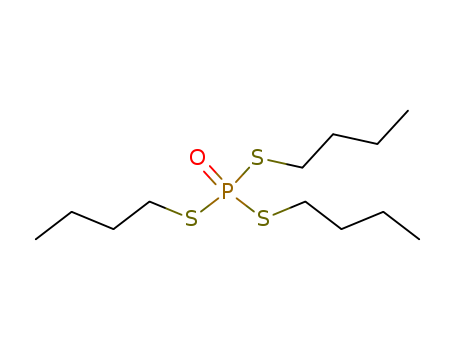
- Chemical Name:Tribufos
- CAS No.:78-48-8
- Molecular Formula:C12H27 O P S3
- Molecular Weight:314.518
- Hs Code.:
- European Community (EC) Number:201-120-8
- UN Number:2811,2902
- UNII:53075G8GRF
- DSSTox Substance ID:DTXSID1024174
- Nikkaji Number:J2.827D
- Wikidata:Q2537076
- Metabolomics Workbench ID:55992
- ChEMBL ID:CHEMBL1901047
- Mol file:78-48-8.mol
Synonyms:butifos;butyl phosphorotrithioate;butyphos;DEF;S,S,S-tributyl phosphorotrithioate;S,S,S-tributyl trithiophosphate;tribufos;tributyl S,S,S-phosphorotrithioate;tributylphosphine oxide;trisbutylphosphine oxide