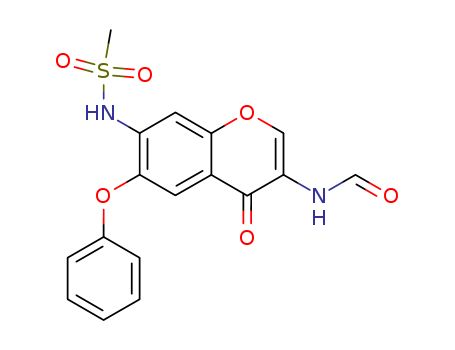
- Chemical Name:Iguratimod
- CAS No.:123663-49-0
- Molecular Formula:C17H14N2O6S
- Molecular Weight:374.374
- Hs Code.:2935009090
- European Community (EC) Number:808-127-0
- UNII:4IHY34Y2NV
- DSSTox Substance ID:DTXSID0048971
- Nikkaji Number:J442.708D
- Wikipedia:Iguratimod
- Wikidata:Q13575264
- NCI Thesaurus Code:C65886
- Metabolomics Workbench ID:153260
- ChEMBL ID:CHEMBL2107455
- Mol file:123663-49-0.mol
Synonyms:3-formylamino-7-methylsulfonylamino-6-phenoxy-4H-1-benzopyran-4-one;T 614;T-614