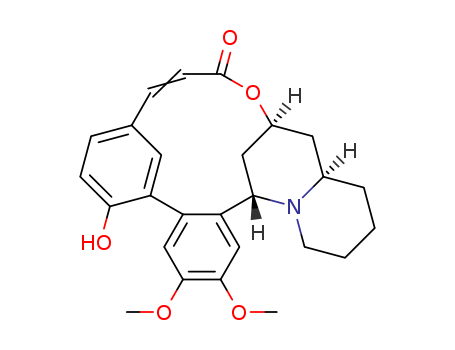
Multi-step reaction with 17 steps
1.1: ethanol / Resolution of racemate; Cooling
2.1: triethylamine / dichloromethane / 20 °C
3.1: phosgene; dimethyl sulfoxide / dichloromethane / 0.33 h / -78 °C / Inert atmosphere
3.2: 4 h / 20 °C / Inert atmosphere
4.1: tetrahydrofuran; diethyl ether / 4.5 h / -78 - 20 °C / Inert atmosphere
5.1: sodium hydrogencarbonate; Dess-Martin periodane / dichloromethane / 2 h / 20 °C
6.1: sodium hydroxide / methanol / 16 h / 55 °C
7.1: trifluoroacetic acid / dichloromethane / 1 h / 0 °C
8.1: sodium hydroxide / tetrahydrofuran / 4 h / 0 - 20 °C
9.1: tetrakis(triphenylphosphine) palladium(0); cesium fluoride / 1,2-dimethoxyethane / 18 h / 95 °C / Inert atmosphere
10.1: L-Selectride / tetrahydrofuran / 1 h / -78 °C
11.1: manganese(IV) oxide / diethyl ether; acetone / 0.33 h
12.1: dmap; triethylamine / dichloromethane / 3 h / 0 - 20 °C
13.1: trifluoroacetic acid / dichloromethane / 3 h / 0 - 20 °C
14.1: dmap; triethylamine / dichloromethane / 3 h / 0 - 20 °C
15.1: nysted reagent; titanium tetrachloride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere
16.1: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / toluene / 16 h / 110 °C / Inert atmosphere
17.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / -30 °C
With
tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride; phosgene; dmap; manganese(IV) oxide; tetrakis(triphenylphosphine) palladium(0); nysted reagent; tetrabutyl ammonium fluoride; titanium tetrachloride; L-Selectride; sodium hydrogencarbonate; Dess-Martin periodane; dimethyl sulfoxide; triethylamine; cesium fluoride; trifluoroacetic acid; sodium hydroxide;
In
tetrahydrofuran; methanol; 1,2-dimethoxyethane; diethyl ether; ethanol; dichloromethane; acetone; toluene;
3.1: Swern oxidation / 3.2: Swern oxidation;
DOI:10.1039/c2ob25880c