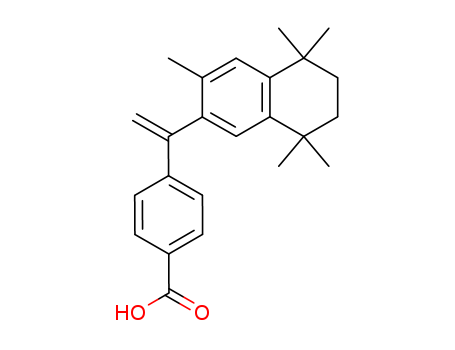
- Chemical Name:Bexarotene
- CAS No.:153559-49-0
- Molecular Formula:C24H28O2
- Molecular Weight:348.485
- Hs Code.:29163990
- European Community (EC) Number:681-650-8
- NSC Number:747528
- UNII:A61RXM4375
- DSSTox Substance ID:DTXSID1040619
- Nikkaji Number:J612.549B
- Wikipedia:Bexarotene
- Wikidata:Q418192
- NCI Thesaurus Code:C1635
- RXCUI:233272
- Pharos Ligand ID:7HWH6ZBVJMRW
- Metabolomics Workbench ID:42694
- ChEMBL ID:CHEMBL1023
- Mol file:153559-49-0.mol
Synonyms:3-methyl-TTNEB;4-(1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethenyl)benzoic acid;bexarotene;LG69 compound;LGD 1069;LGD-1069;LGD1069;Targretin