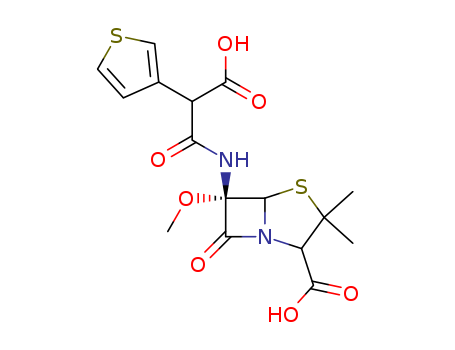
- Chemical Name:Temocillin
- CAS No.:66148-78-5
- Molecular Formula:C16H18N2O7S2
- Molecular Weight:414.46
- Hs Code.:
- European Community (EC) Number:266-184-1
- UNII:03QB156W6I
- DSSTox Substance ID:DTXSID201009398
- Nikkaji Number:J21.367E
- Wikipedia:Temocillin
- Wikidata:Q3983108
- NCI Thesaurus Code:C76858
- Metabolomics Workbench ID:57904
- ChEMBL ID:CHEMBL1276310
- Mol file:66148-78-5.mol
Synonyms:BRL 17421;disodium 6beta-(2-carboxy-2-thien-3-ylacetamido)-6alpha-methoxypenicillanate;temocillin;temocillin, (2S-(2alpha,5alpha,6alpha))-isomer