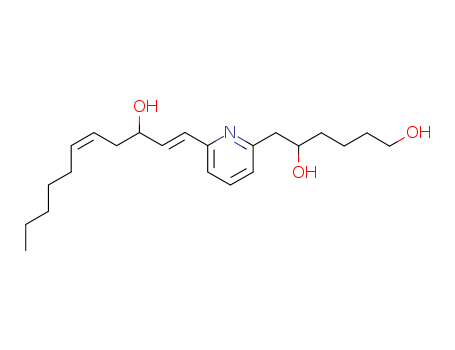
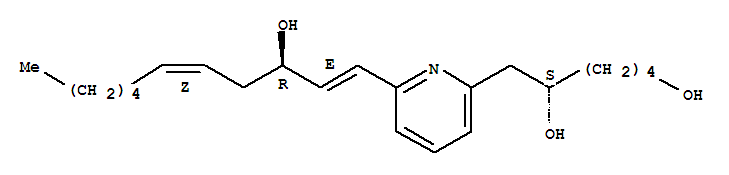
- Chemical Name:U-75302
- CAS No.:119477-85-9
- Molecular Formula:C22H35NO3
- Molecular Weight:361.525
- Hs Code.:
- European Community (EC) Number:634-249-7
- ChEMBL ID:CHEMBL110037
- Mol file:119477-85-9.mol
Synonyms:6-(6-(3-hydroxy-1,5-undecadien-1-yl)-2-pyridinyl)-1,5-hexanediol;U 75302;U-75302;U75302


 Xi
Xi

