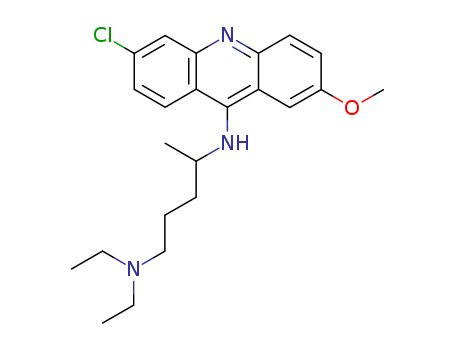
- Chemical Name:Quinacrine
- CAS No.:83-89-6
- Deprecated CAS:66777-81-9
- Molecular Formula:C23H30ClN3O
- Molecular Weight:399.964
- Hs Code.:
- European Community (EC) Number:200-700-8,201-508-7
- UNII:H0C805XYDE
- DSSTox Substance ID:DTXSID7022627
- Nikkaji Number:J3.877F
- Wikipedia:Mepacrine
- Wikidata:Q417208
- NCI Thesaurus Code:C87656
- Pharos Ligand ID:YNBYCUC8A8TY
- Metabolomics Workbench ID:145826
- ChEMBL ID:CHEMBL7568
- Mol file:83-89-6.mol
Synonyms:Acrichine;Atabrine;Atebrin;Dihydrochloride, Quinacrine;Dimesylate, Quinacrine;Hydrochloride, Quinacrine;Mepacrine;Monoacetate, Quinacrine;Monohydrochloride, Quinacrine;Monomesylate, Quinacrine;Quinacrine;Quinacrine Dihydrochloride;Quinacrine Dihydrochloride, Dihydrate;Quinacrine Dihyrochloride, (R)-Isomer;Quinacrine Dihyrochloride, (S)-Isomer;Quinacrine Dimesylate;Quinacrine Hydrochloride;Quinacrine Monoacetate;Quinacrine Monohydrochloride;Quinacrine Monomesylate;Quinacrine, (+-)-Isomer;Quinacrine, (R)-Isomer;Quinacrine, (S)-Isomer