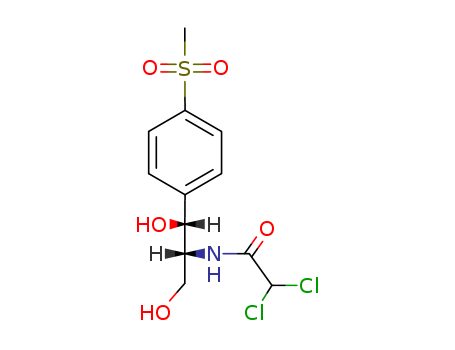
- Chemical Name:Thiamphenicol
- CAS No.:15318-45-3
- Deprecated CAS:14786-51-7,32430-04-9,3785-14-6,90-91-5
- Molecular Formula:C12H15Cl2NO5S
- Molecular Weight:356.227
- Hs Code.:29414000
- European Community (EC) Number:239-355-3
- NSC Number:758396
- UNII:FLQ7571NPM,283383NO13
- DSSTox Substance ID:DTXSID5021338
- Nikkaji Number:J9.286J
- Wikipedia:Thiamphenicol
- Wikidata:Q425015
- NCI Thesaurus Code:C61920,C61969
- Metabolomics Workbench ID:54518
- ChEMBL ID:CHEMBL1236282
- Mol file:15318-45-3.mol
Synonyms:Dextrosulfenidol;Raceophenidol;Thiamcol;Thiamphenicol;Thiomycetin;Thiophenicol;Urfamycin;Vicemycetin