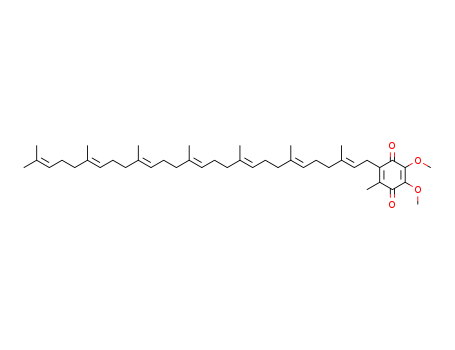
- Chemical Name:Ubiquinone 7
- CAS No.:303-95-7
- Molecular Formula:C44H66 O4
- Molecular Weight:659.006
- Hs Code.:
- NSC Number:147790
- UNII:RRK47DEG6Q
- DSSTox Substance ID:DTXSID501318261
- Nikkaji Number:J1.205.855A,J11.403K
- Wikidata:Q27120651
- NCI Thesaurus Code:C87475
- Metabolomics Workbench ID:56658
- Mol file:303-95-7.mol
Synonyms:coenzyme Q7;CoQ7;ubiquinone 7;ubiquinone 7, (Z,Z,Z,E,E,E)-isomer