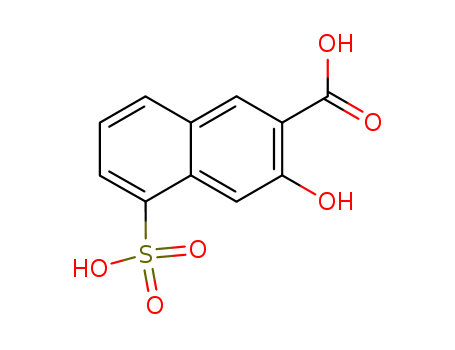
- Chemical Name:3-Hydroxy-5-sulfo-2-naphthoic acid
- CAS No.:86-64-6
- Molecular Formula:C11H8 O6 S
- Molecular Weight:268.247
- Hs Code.:2918290000
- European Community (EC) Number:201-688-7
- DSSTox Substance ID:DTXSID001006574
- Nikkaji Number:J192.638A
- Wikidata:Q83002332
- Mol file:86-64-6.mol
Synonyms:3-Hydroxy-5-sulfo-2-naphthoic acid;86-64-6;3-Hydroxy-5-sulpho-2-naphthoic acid;EINECS 201-688-7;NSC 40597;NSC40597;6313-96-8;3-hydroxy-5-sulfonaphthalene-2-carboxylic acid;C11H8O6S;SCHEMBL1805368;3-hydroxy-5-sulfo-2-naphthoesyre;DTXSID001006574;C11-H8-O6-S;3-Hydroxy-5-sulfo-2-naphthalenecarboxylic acid;3-hydroxy-5-sulfo-naphthalene-2-carboxylic acid;2-Naphthalenecarboxylic acid, 3-hydroxy-5-sulfo-