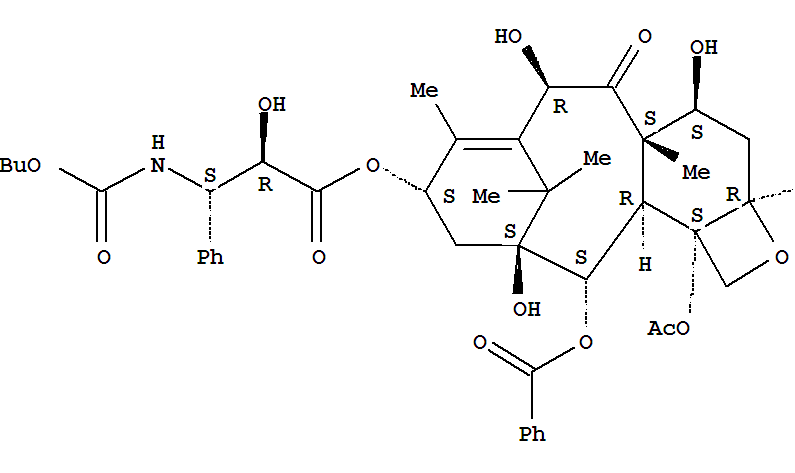
- Chemical Name:Docetaxel
- CAS No.:114977-28-5
- Deprecated CAS:129522-72-1,216252-50-5,216252-50-5
- Molecular Formula:C43H53NO14
- Molecular Weight:807.892
- Hs Code.:2932999099
- European Community (EC) Number:601-339-2
- NSC Number:628503
- UNII:699121PHCA
- DSSTox Substance ID:DTXSID0040464
- Nikkaji Number:J314.049K
- Wikipedia:Docetaxel
- Wikidata:Q420436
- NCI Thesaurus Code:C61734
- RXCUI:1299922
- Pharos Ligand ID:UHFR3MZHPXJJ
- Metabolomics Workbench ID:43456
- ChEMBL ID:CHEMBL92
- Mol file:114977-28-5.mol
Synonyms:docetaxel;docetaxel anhydrous;docetaxel hydrate;docetaxel trihydrate;docetaxol;N Debenzoyl N tert butoxycarbonyl 10 deacetyltaxol;N-debenzoyl-N-tert-butoxycarbonyl-10-deacetyltaxol;NSC 628503;RP 56976;RP-56976;RP56976;Taxoltere metro;Taxotere


 Xi
Xi


