10.1021/acs.orglett.9b03022
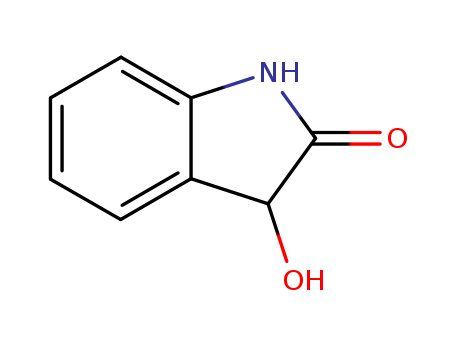
The research explores a novel synthetic method to produce a broad range of unnatural C2-quaternary 2-hydroxyindoxyls using a one-pot domino Grignard addition and oxidation process. The purpose of this study is to address the challenge of synthesizing 2-hydroxyindoxyls, which are less explored but significant due to their presence in various natural products and biologically active compounds. The key chemicals involved include 3-hydroxyoxindoles as starting materials and various Grignard reagents such as phenylmagnesium bromide. The reaction proceeds via a 1,2-hydride shift followed by autoxidation, yielding 2-hydroxyindoxyls in high yields. The study concludes that this method offers a simple, scalable, and operationally easy approach to synthesizing a wide range of 2-hydroxyindoxyls with different functional groups. Additionally, the synthesized 2-hydroxyindoxyls can serve as precursors for further transformations, such as the synthesis of bis-indoxyl spirofurans and differentially substituted 3-oxindoles, demonstrating the versatility and potential of this synthetic protocol in organic chemistry.