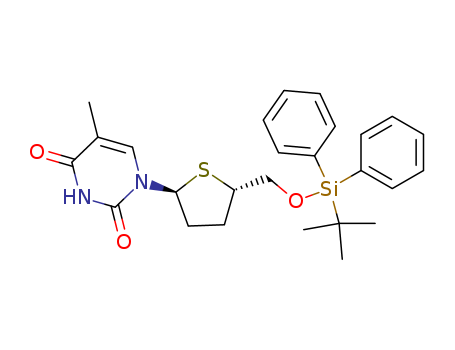
- Chemical Name:1-<5-O-(tert-butyldiphenylsilyl)-2,3-dideoxy-4-thio-α-D-ribofuranosyl>thymine
- CAS No.:137719-37-0
- Molecular Formula:C26H32N2O3SSi
- Molecular Weight:480.703
- Hs Code.:
- Mol file:137719-37-0.mol
Synonyms:1-<5-O-(tert-butyldiphenylsilyl)-2,3-dideoxy-4-thio-α-D-ribofuranosyl>thymine