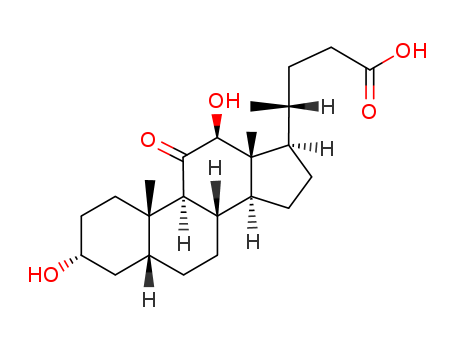
- Chemical Name:3alpha,12beta-Dihydroxy-11-oxo-5beta-cholan-24-oic Acid
- CAS No.:15173-30-5
- Molecular Formula:C24H38 O5
- Molecular Weight:406.563
- Hs Code.:
- NSC Number:66587
- Wikidata:Q76085431
- Metabolomics Workbench ID:36429
- Mol file:15173-30-5.mol
Synonyms:3alpha,12beta-Dihydroxy-11-oxo-5beta-cholan-24-oic Acid;15173-30-5;NSC66587;(4R)-4-[(3R,5R,8S,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethyl-11-oxo-1,2,3,4,5,6,7,8,9,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]pentanoic acid;CHEBI:186602;LMST04010188;NSC-66587