10.1016/S0040-4039(01)91879-9
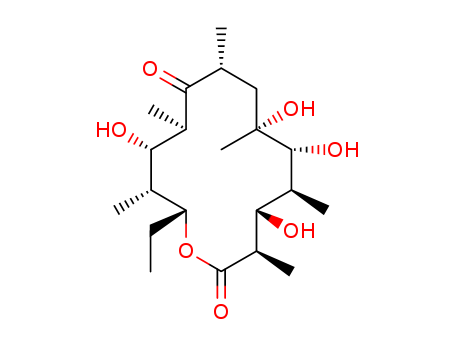
The study details the synthesis of 5-acetoxy-9-oxotridecanolactone as a model for erythronolide B, the aglycone of erythromycin B. The researchers aimed to develop a route to 5-acetoxy-13-hydroxy-9-oxotridecanoic acid, with the intention of lactonization at the final stage of macrolide synthesis. The synthesis involved assembling a thirteen-carbon backbone from two cyclopentanoid and one propanoid unit. Key steps included alkylation of the potassio salt of cyclopentanone carboxylic ester with 1,3-dibromopropane, decarboxylation with HBr, Baeyer-Villiger oxidation using CF3CO3H, and further alkylation and decarboxylation steps. The study also involved protecting and unmasking the cyclopentanone carbonyl group, reduction of an ester group with LiAlH4, and oxidation with Collins' reagent. The final lactonization was achieved using p-toluenesulfonyl chloride and Et3N, or alternatively, 1,1'-carbonyldiimidazole and sodium t-amylate. The synthetic route demonstrated potential applicability to erythronolide and other macrolide systems.