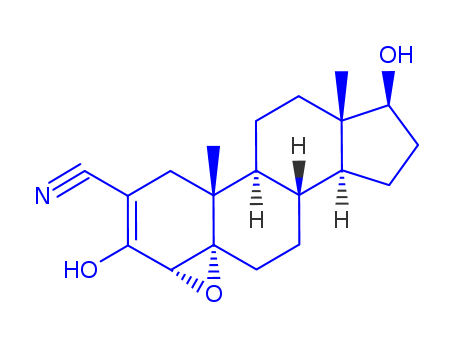
- Chemical Name:Trilostane
- CAS No.:13647-35-3
- Deprecated CAS:27107-98-8,28414-46-2
- Molecular Formula:C20H27NO3
- Molecular Weight:329.439
- Hs Code.:29372900
- European Community (EC) Number:237-133-0
- UNII:L0FPV48Q5R
- DSSTox Substance ID:DTXSID9023706
- Nikkaji Number:J108.016D
- Wikipedia:Trilostane
- Wikidata:Q907313
- NCI Thesaurus Code:C1263
- RXCUI:38668
- Pharos Ligand ID:4BK6B8JNKQNX
- Metabolomics Workbench ID:43337
- ChEMBL ID:CHEMBL1200907
- Mol file:13647-35-3.mol
Synonyms:4alpha,5-epoxy-17beta-hydroxy-3-oxoandrostane-2-carbonitrile;Modrenal;trilostane;WIN 24,540