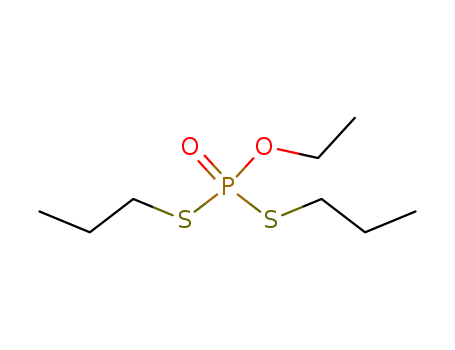
- Chemical Name:Ethoprophos
- CAS No.:13194-48-4
- Molecular Formula:C8H19O2PS2
- Molecular Weight:242.343
- Hs Code.:29309090
- European Community (EC) Number:236-152-1
- ICSC Number:1660
- UN Number:3018
- UNII:765Y5683OQ
- DSSTox Substance ID:DTXSID4032611
- Nikkaji Number:J8.092F
- Wikipedia:Ethoprophos
- Wikidata:Q411754
- Metabolomics Workbench ID:55958
- ChEMBL ID:CHEMBL1894994
- Mol file:13194-48-4.mol
Synonyms:ethoprop;ethoprophos;Mocap;O-ethyl S,S-diprophyl phosphorodithioate


 T+,
T+, N
N

