- Chemical Name:Lime
- CAS No.:1305-78-8
- Deprecated CAS:104624-96-6,245321-52-2,60873-85-0
- Molecular Formula:CaO
- Molecular Weight:56.08
- Hs Code.:28259019
- European Community (EC) Number:277-225-8
- UNII:C7X2M0VVNH
- DSSTox Substance ID:DTXSID5029631
- Mol file:1305-78-8.mol
Synonyms:calciumoxide;calcium;oxygen(2-);Lime oxide;73018-51-6;MFCD00010911;calcium oxid;LIME [HSDB];CALCIUM OXIDE [MI];CALCIUM OXIDE [FCC];CALCIUM OXIDE [JAN];CALCIUM OXIDE [INCI];LIME [USP MONOGRAPH];CALCIUM OXIDE [MART.];CALCIUM OXIDE [WHO-DD];DTXSID5029631;BRPQOXSCLDDYGP-UHFFFAOYSA-N;LIME (CALCIUM OXIDE) [II];EINECS 277-225-8;AKOS032949954;ETHYLBIS(2-BROMOETHYL)CARBAMATE;FT-0604488;1,6-Octadien-3-ol, 3,7-dimethyl-, acid isomerized;1,6-Octadien-3-ol, 3,7-dimethyl-, acid-isomerized


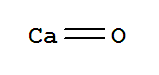
 C,
C, Xi
Xi
